Single-wall carbon nanotubes bearing covalently linked phthalocyanines - Photoinduced electron transfer
Abstract
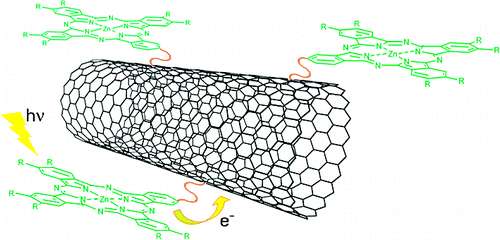
HiPco single-walled carbon nanotubes (SWNTs) have been sidewall-functionalized with phthalocyanine addends following two different approaches: a straightforward Prato reaction with $N$-octylglycine and a formyl-containing phthalocyanine, and a stepwise approach that involves a former Prato cycloaddition to the double bonds of SWNTs using $p$-formyl benzoic acid followed by esterification of the derivatized nanotubes with an appropriate phthalocyanine molecule. The two materials obtained by these routes comprise different carbon/Pc-addenda ratios, as evidenced by Raman, TGA, and photophysical studies. The occurrence of electron transfer from photoexcited phthalocyanines to the nanotube framework in these ZnPc-SWNT ensembles is observed in transient absorption experiments, which confirm the absorption of the one-electron oxidized ZnPc cation and the concomitant bleaching of the van Hove singularities typical from SWNTs. Charge-separation (i.e., $2.0 \times 10^{10}$ s$^{-1}$) and charge-recombination (i.e., $1.5 \times 10^6$ s$^{-1}$) dynamics reveal a notable stabilization of the radical ion pair product in dimethylformamide.
Full citation
B. Ballesteros, G. de la Torre, C. Ehli, G. M. A. Rahman, F. Agulló-Rueda, D. M. Guldi, and T. Torres,
“Single-wall carbon nanotubes bearing covalently linked phthalocyanines - Photoinduced electron transfer,”
J. Am. Chem. Soc. 129, 5061–5068 (2007).
DOI: 10.1021/ja068240n